Abstract
Background/objectives
To examine prevalence of failed visual assessment at 8–10 years in children born to methadone-maintained opioid dependent (MMOD) mothers and relate this to known in utero substance exposure.
Subjects/methods
Follow up of observational cohort study of methadone-exposed and comparison children matched for birthweight, gestation and postcode of residence at birth. Participants were 144 children (98 exposed, 46 comparison). Prenatal drug exposure was previously established via comprehensive maternal and neonatal toxicology. Children were invited to attend for visual assessment and casenotes were reviewed. Presence of acuity poorer than 0.2 logMAR, strabismus, nystagmus and/or impaired stereovision constituted a ‘fail’. Fail rates were compared between methadone-exposed and comparison children after adjusting for known confounding variables.
Results
33 children attended in person: data were also derived from casenote review for all children. After controlling for maternal reported tobacco use, methadone-exposed children were more likely to have a visual ‘fail’ outcome, adjusted odds ratio 2.6, 95% CI 1.1–6.2; adjusted relative risk 1.8 (95% CI 1.1–3.4). Visual ‘fail’ outcome rates did not differ between methadone-exposed children who had (n = 47) or had not (n = 51) received pharmacological treatment for neonatal abstinence/opioid withdrawal syndrome (NAS/NOWS); fail rate 62% vs 53% (95% CI of difference—11–27%).
Conclusions
Children born to MMOD mothers are almost twice as likely as unexposed peers to have significant visual abnormalities at primary school age. Prenatal methadone exposure should be considered in the differential diagnosis of nystagmus. Findings support visual assessment prior to school entry for children with any history of prenatal opioid exposure.
Trial registration
The study was prospectively registered on ClinicalTrials.gov (NCT03603301), https://clinicaltrials.gov/ct2/show/NCT03603301.
Similar content being viewed by others
Introduction
Opioid use disorder (OUD) in pregnancy causes significant harm [1, 2]. Harm reduction policies, including maintenance treatment with methadone and/or other opioids, improve engagement with antenatal care and reduce risk-taking behaviours and preterm birth [1, 3, 4]. Prenatal opioid exposure may lead to neonatal abstinence/opioid withdrawal syndrome (NAS/NOWS); causation of longer-term sequelae for these children is difficult to ascertain due to confounding effects of often poorly documented polydrug misuse, tobacco smoking, alcohol and challenged home environment [5,6,7,8,9].
Prenatal opioid exposure has been associated with problems suggesting impaired development of vision [6, 10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] including strabismus and nystagmus; reports approximately mirror global opioid epidemics [3, 37,38,39]. In a cohort of healthy infants born to methadone-maintained opioid dependent (MMOD) mothers, for whom extensive toxicology data were available, altered neonatal visual evoked potentials (VEP), an objective physiological marker of integrity and maturity of the visual pathway, were independently associated with prenatal methadone exposure [40]. Vision was impaired at six months of age, as well as general development [41, 42]. We now report follow-up data from this cohort at ages 8–10 years with the aims of comparing vision fail outcome rates between exposed and comparison children after controlling for confounding variables, and relating visual outcomes to prenatal substance exposure.
Methods
Design
This observational cohort study investigated visual outcomes of prenatally methadone-exposed children compared with comparison children, aged 8–10 years. The study was pre-registered at ClinicalTrials.gov, NCT 03603301.
Participants
The cohort was previously recruited at birth and investigated at 1–3 days [40] and at 6–7 months of age [41]. Eligible exposed infants were born to MMOD mothers after 36 completed weeks’ gestation without congenital ocular abnormality or significant neonatal illness. Prenatal drug exposure was determined via comprehensive toxicology (maternal urine, infant urine and meconium), maternal casenote review and confidential interview [40]. Comparison infants were born contemporaneously (2008–10) at the same maternity hospital, matched for completed week of gestation, birthweight (±250 g) and material deprivation. Maternal postcode of residence at delivery was used to define the 2001 Carstairs material deprivation category (±1, 1–7 from least to most deprivation based on four indicators judged to represent material disadvantage (lack of car ownership, low occupational social class, overcrowded households and male unemployment) [43]). As far as possible, comparison infants were also matched for maternal tobacco use. A subgroup of comparison infants had meconium drug analysis and a subset of meconium samples from both groups was analysed for prenatal alcohol exposure. The exposed cohort size (n = 102, of whom 100 completed neonatal VEPs) was chosen for sufficient VEP parameter precision to distinguish exposed infants who developed NAS/NOWS (defined as receiving pharmaceutical treatment as per well-established hospital protocol) from those who did not [11]. Comparison cohort size (n = 51, of whom 50 completed neonatal VEPs) was chosen for pragmatic reasons given the time commitment required for neonatal testing and 6–7 month follow up. Selection bias was low due to the high consent rate (98%) at infant recruitment [40].
For follow-up at 8–10 years, families of all recruited children, including three who did not complete neonatal VEPs, were invited to attend for investigation. Initial contact was by letter asking families to opt in or out via a return slip; most recent home address was sought from general practitioners if there was no response. Follow-up phone calls were made to families who opted in and to non-responders.
Measures
Children attending in person were assessed at the paediatric Clinical Research Facility, Queen Elizabeth University Hospital, Glasgow. This healthcare complex includes the regional children’s hospital and the regional paediatric ophthalmology service. Written informed consent was given by the accompanying adult; children gave written informed assent. Care status (living with birth parent(s), kinship carer, foster carer or adoptive parent(s)) as well as diagnoses of autistic spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD), and/or foetal alcohol spectrum disorder (FASD) were noted and details of any previous attendance at ophthalmology clinics were scrutinised. Following detailed visual assessment (see supplementary material), children were ascribed a ‘pass’ or ‘fail’ result based on pre-determined fail criteria including one or more of: acuity poorer than 0.2 logMAR not attributable to refractive error; manifest but not latent strabismus; nystagmus; abnormal stereovision. Visual assessment pass/fail was the primary outcome measure. To quantify the extent of visual problems a novel, composite ‘visual detriment index’ (VDI) was created for the study as a secondary outcome measure. Severity of fail criteria were scored (nystagmus = 3, strabismus = 2, impaired binocular vision = 2, poor acuity = 2) and summed, giving possible scores 0–9. A higher score was attributed to nystagmus because of its relatively higher detriment to vision and to reflect how uncommon it is in the population at large.
To avoid bias due to any comprehension difficulties, vision tests were selected to be easily performed by primary school age children. Researchers were masked to exposure status to limit bias potential. Reporting bias (carers of children with eye problems more likely to attend) would likely affect both exposed and comparison families similarly.
Children failing visual assessment or causing concern not already being addressed were notified to relevant services after discussion with their carer. Families were offered cash to cover expenses and a £20 child’s gift voucher.
For children who did not attend for follow up (no contact details, family did not respond to invitation, actively declined to attend in person or failed to attend arranged assessment(s)), casenotes were reviewed for record of any hospital eye service findings. Casenote review is a robust process due to two factors; 1) mandated use of the Community Health Index number in NHS Scotland which enables linking of health data for research purposes and 2) comprehensive, electronic-only patient health records for both acute and community settings. The same pre-determined vision ‘fail’ criteria were used and both care status and diagnoses of ASD, ADHD, and/or FASD noted if available. Any non-attending child confirmed to be living within the health board region (NHS Greater Glasgow & Clyde) at the time of casenote review but not ever referred to hospital eye services was attributed a ‘pass’ result. The Scottish universal pre-school vision screening programme ensures a high identification rate of visual problems (89% coverage for 2008–10 births) and subsequent referral to regional services. The likelihood of a ‘pass’ result being incorrectly attributed to a non-attending child was therefore felt to be low. Casenote review was also undertaken for children who attended in person.
Analyses
Characteristics and outcomes of attendees versus non-attendees were compared to assess any bias associated with in-person prospective data collection versus data collected via retrospective casenote review. Agreement on pass/fail outcome between casenote review and in-person assessment was compared for attending children.
Visual assessment pass/fail was presented as an unadjusted odds ratio (ratio of fail outcomes, exposed vs comparisons, to ratio of pass outcomes, exposed vs comparisons). Odds ratio is a poor approximation of the more intuitive relative risk (risk ratio) for outcomes which are not rare [44] and so unadjusted relative risk was also calculated. To identify potentially confounding variables, maternal, birth and neonatal characteristics were compared between exposed and comparison groups. Methadone exposure and potentially confounding variables were treated as independent dichotomous variables (non-exposed as the reference category) in a multivariable logistic regression analysis with pass/fail visual outcome as the dependent variable, and in a multivariable linear regression analysis with VDI as the dependent variable. For exposed children only, visual outcomes were compared by drug exposure group, by presence or absence of (treated) NAS/NOWS and by prescribed maternal methadone dose at birth. Multivariable linear regression was used to examine the relationship between each additional drug exposure and VDI. Sex and care status were treated as potential modifiers for sub-group analysis. Based on the series of three research investigations of this cohort, the positive and negative predictive value of the first investigation (abnormal neonatal VEPs [40]) and the second investigation (a failed or borderline visual assessment at 6–7 months [41]) were calculated.
Analyses were performed using Minitab v20.3 (Mintab LLC, PA, USA) and MedCalc® v20.014 (MedCalc Software Ltd, Ostend, Belgium). The study was approved by West of Scotland Research Ethics Committee 3 (17/WS/0093).
Results
Participants
Of the whole cohort of 153 children, eight were untraceable; one further child was excluded due to a diagnosis of retinal dystrophy. Of the remaining 144 children (98 exposed, 46 comparison), 67 (46%) did not respond to invitations, 26 (18%) declined to attend in person, 18 (13%) did not show and 33 (23%) attended. Data were therefore derived from casenote review alone for 111 children (77 exposed, 34 comparison) and from findings at attendance as well as casenote review for 33 (21 exposed, 12 comparison) children (supplementary material Fig. S1). 45 of the 111 non-attending children (27 exposed, 18 comparison) were still resident in the health board region but had not ever been referred to its hospital eye services and thereby were attributed a ‘pass’ result.
Attendees and non-attendees did not differ in terms of birth characteristics, prenatal drug exposure, demographics or visual outcomes at last documented follow-up. Age at assessment was older for attendees than age at most recent hospital eye service attendance or other healthcare encounter for non-attendees (shown for exposed and comparison groups, supplementary material Table S1). Social care differed markedly between exposed and comparison children; over half (49/93) of exposed children no longer lived with either birth parent. Fourteen MMOD but no comparison mothers had died (Table 1). Two exposed children were homeless.
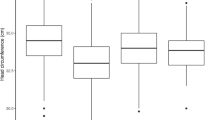
Maternal reported tobacco use differed significantly between exposed (93/98, 95%) and comparison children (25/46, 54%, Table 1) and was therefore treated as a potentially confounding variable. Head circumference at birth also differed significantly between exposed and comparison children (Table 1), but since prenatal opioid exposure is associated with reduced fetal head growth [6, 44], head circumference at birth was not treated as a confounding variable. Only 17/98 exposed children were prenatally exposed solely to opioids (drug group 1, Fig. 1, Table 2); for nine, this was exclusively prescribed methadone.
Opioid is methadone ± opiates; BDZ, benzodiazepines; stimulants are cocaine and/or amphetamines. Drug groups: 1) opioids alone (n = 17); 2) opioids + cannabinoid (n = 12); 3) opioids + benzodiazepine (n = 14); 4) opioids + benzodiazepine + cannabis (n = 30); 5) opioids + stimulants ± benzodiazepine or cannabis (n = 25). Opiates most likely illicit heroin. If infant or postnatal maternal urine was positive for opiates and opioid analgesia in labour was documented, in the absence of declared illicit maternal opiate or positive prenatal maternal urine, infant was not considered exposed.
Visual findings
Visual pass/fail outcomes from in-person assessment and from casenote review matched for all 33 (100%) children attending for assessment. Children born to MMOD mothers were more likely than comparison children to have a visual ‘fail’ outcome: 56/98 vs 12/46 (Table 1). The unadjusted odds ratio, 3.8 (95% CI 1.8–8.2, p = 0.001), indicates children failing vision assessment were about four times more likely to have been born to MMOD mothers than children who passed. The unadjusted relative risk, 2.2 (95% CI 1.3–4.0), indicates that children born to MMOD mothers were over twice as likely to fail vision assessment. Exposed children were also more likely to have attended or be attending hospital eye services and had higher median VDI (Table 1). Twenty (20%, 95% CI 13–30%) exposed children had nystagmus, none with an explanatory diagnosis or family history. Sixteen of these 20 had previously been assessed at 6–7 months: nystagmus was evident in only eight (50%) at that age [41]. Six of these 20 children with nystagmus attended in person; eye movement recordings (supplementary material) showed waveforms consistent with fusion maldevelopment nystagmus syndrome. No comparison child had nystagmus.
After controlling for reported maternal tobacco use, children born to MMOD mothers were more likely to have a visual fail outcome (adjusted odds ratio 2.6, 95% CI 1.1–6.2, Table 3A). The adjusted relative risk of children born to MMOD mothers failing visual assessment at 8–10 years was 1.8 (95% CI 1.1–3.4). Similarly, being born to a MMOD mother was associated with significantly higher VDI after controlling for maternal tobacco use, with an adjusted effect size of 1.4 (95% CI 0.45–2.36) (Table 3B).
For children born to MMOD mothers, VDI did not differ by drug-exposure group (Kruskal-Wallis test, H = 6.3, df = 4, p = 0.18). The 17 opioid-only exposed children (drug group 1) with visual fail outcomes had similar findings to the exposed group as a whole (Fig. 2).
Left, by drug exposure group: 1) opioids alone (n = 17); 2) opioids + cannabinoid (n = 12); 3) opioids + benzodiazepine (n = 14); 4) opioids + benzodiazepine + cannabis (n = 30); 5) opioids + stimulants (cocaine and/or amphetamines) ± benzodiazepine or cannabis (n = 25). Right, by neonatal abstinence/opioid withdrawal syndrome (NAS/NOWS) treatment category (n = 47 treated, n = 51 not treated).
Visual fail outcome rates did not differ between exposed children who had (29/47, 62%) or had not (27/51, 53%) been treated for NAS/NOWS (95% CI of difference -11–27%, χ2 0.8, df = 1, p = 0.4) and neither did VDI differ between children who had or had not been treated for NAS/NOWS (median 2 vs 2, Mann-Whitney W = 2527, p = 0.16, Fig. 2).
MMOD mothers of children failing visual assessment had been prescribed higher daily methadone doses at delivery than mothers of children who passed (57.5 vs 45 mg per day, 95% CI of difference 5–27 mg, Mann-Whitney 3008, p = 0.016). Prescribed maternal methadone dose at delivery was weakly and positively correlated with VDI (rho 0.31, 95% CI 0.11–0.49, p = 0.002).
Multivariable ordinal regression modelling of additional drug exposure(s) found only benzodiazepine to be independently associated with higher VDI (Table 3C), suggesting that prenatal benzodiazepine in addition to methadone exposure slightly but significantly further impairs visual outcomes.
Considering the whole cohort, a similar proportion of visual fail outcomes was found for male (35/66, 53%) and female (33/78, 42%) children (95% CI of difference −6–26%, p = 0.20). For children born to MMOD mothers, similar proportions of visual fail outcomes were found for children in kinship, foster or adoptive care (30/48, 63%) and for children living with a birth parent (24/44, 55%) (95% CI of difference −12–27%, p = 0.44): care status was therefore unlikely to be a significant modifier.
From the series of three research investigations of this cohort, 140 children had both neonatal VEP data and visual findings at 8–10 years: abnormal neonatal VEPs [40] had positive and negative predictive values for failed childhood visual outcome of 77 (61–88)% and 73 (60–84)% respectively. For the 102 children with both 6–7 month and 8–10 year visual findings, a failed or borderline result at 6–7 months [41] had lower positive and negative predictive values for failed mid-childhood visual outcome, 56 (42–69)% and 59 (48–70)%, respectively. Sixteen infants passed at 6–7 months but subsequently had a visual fail outcome childhood assessment, including three children with nystagmus.
Discussion
Within this cohort, after adjusting for maternal tobacco use in pregnancy, being born to a MMOD mother almost doubled the risk of children failing visual assessment at 8–10 years. Almost half of exposed children had strabismus, conservatively 10-fold the expected prevalence of 2–3.4% in UK children [45, 46]. One in five exposed children had nystagmus, conservatively 300-fold the expected prevalence in children free of neurological or retinal disease [47]. Poorer outcomes were associated with higher prescribed maternal methadone dose and visual problems were equally frequent in children who did and did not receive treatment for NAS/NOWS. Research assessments of this cohort at 6–7 months [40, 41] were poorly predictive of childhood visual outcomes. However, all children with fail outcomes at 8–10 years had already been identified via routine healthcare provision.
More than half of children born to MMOD mothers with a visual fail outcome at 8–10 years had strabismus and/or nystagmus; this is more common than reported elsewhere, which may reflect higher ascertainment with universal access to pre-school vision screening. The high rate of strabismus even in our comparison children (13%) may be explained at least partly by maternal tobacco use. By comparison, 47% of exposed children had strabismus; the marked difference between the groups persisted even after controlling for maternal tobacco smoking. An alarmingly high proportion—20%—of exposed children had nystagmus with a waveform consistent with fusion maldevelopment nystagmus syndrome, suggesting impaired cortical visual input to subcortical vestibular pathways [48]. This may imply a teratogenic effect of methadone (and/or other substances of misuse) upon the striate cortex and connective tracts, reducing the number and/or connectivity of binocular connections [49, 50] which could explain both strabismus and fusion maldevelopment nystagmus syndrome [51]. Additional exposure to benzodiazepine was associated with more visual problems, suggesting that benzodiazepine slightly but significantly further impairs visual outcomes. This association did not hold for additional opiates, cannabis, cocaine or amphetamines.
A teratogenic effect of prenatal opioid exposure upon the developing fetal brain is both plausible and supported by evidence including smaller head size at birth and neonatal brain MRI showing loss of connective tracts [50], multiple observational studies [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26], the albeit weak dose-response relationship with prescribed methadone dose at delivery in the current study, and evidence from animal models [52, 53]. Meta analyses also strongly suggest an association between prenatal methadone exposure and impaired childhood neurodevelopmental outcomes [5,6,7]. While all of these data support an association between prescription of methadone to opioid-dependent pregnant women and impaired childhood visual outcomes, they do not prove causation [36].
A cautious conclusion from this study is that children born to MMOD mothers are up to twice as likely as unexposed peers to have visual abnormalities by mid-primary school age. As previously noted in a much larger cohort, a history of NAS/NOWS is not required for the child to be at risk of longer term harm [32]. Current guidelines [1, 4] state that methadone is safe in pregnancy other than the risk of (transient) NAS/NOWS: while methadone maintenance therapy improves pregnancy outcomes, pregnant mothers and their caregivers need to balance management of OUD with consideration of the risk of longer term problems for the unborn child [32], which are not currently described in guidelines. Strabismus and especially nystagmus confer learning and psychosocial difficulties [54], additional burdens on children with reduced life opportunities [55].
From the visual findings described in this study and by others, as well as other neurodevelopmental problems [5, 6, 32], we propose a fetal opioid spectrum disorder and suggest that prenatal opioid exposure be considered in the differential diagnosis of infantile and/or childhood nystagmus. Even if children born to MMOD mothers remained well in the neonatal period, they should have formal visual assessment post-infancy and prior to school entry with emphasis on strabismus, binocular vision and nystagmus. Consideration of further detailed visual assessment is warranted, particularly if there are educational and/or behavioural difficulties. All health professionals caring for children have a role in ensuring these vulnerable children are assessed, especially when universal childhood visual screening programmes are lacking. Studies to determine the longer term safety of methadone alternatives, such as buprenorphine, should include visual follow up [24, 56,57,58] and should control for maternal tobacco use.
Strengths of this study include detailed knowledge of prenatal drug exposure, prospective design and a comparison group matched at recruitment for gestation, birthweight and deprivation. The additional challenges of drug misuse mean that postcode of residence is not a perfect proxy for socioeconomic deprivation, and tobacco smoking is also very difficult to control for. A major limitation of the study was the small proportion (23%) of the cohort attending for assessment. Significant efforts were made to contact families, the vast majority of whom remained resident in the Glasgow area. However, researching some of the most materially-deprived families in Scotland, many of whom have chaotic, unpredictable lifestyles and do not engage well with healthcare or perceived authority, is challenging and therefore in-person investigation of 33 of the traceable cohort of 144 children represents a substantial achievement. Furthermore, casenote review proved to be robust, matching in-person findings in terms of the primary outcome measure, visual assessment pass/fail. Tobacco use may be underestimated due to maternal self-report but with 95% reported use by MMOD mothers, this is likely to have applied only to comparison mothers of whom 54% reported tobacco use. Prenatal alcohol exposure was assessed by meconium analysis of only a subgroup of both exposed and comparison children, meaning less reliable assessment of this confounding factor. Assuming a ‘pass’ result for non-attending children confirmed to be living locally but not ever referred to hospital eye services may bias the findings, but bias is likely to be limited given the high retention of local residency and high coverage by the national pre-school universal vision screening programme. Since our cohort included exclusively a treatment population (children of MMOD mothers), our findings are not generalisable to children of other opioid users such as unsupported opiate users or those using licit opioids.
Being born to an MMOD mother is strongly associated with visual problems in mid-childhood. Pregnant women with OUD and their caregivers need to consider the potential risk of visual abnormalities and other developmental disorders in the unborn child. Prenatal opioid exposure should be considered in the differential diagnosis of nystagmus and, regardless of whether NAS/NOWS was manifest, children born to MMOD mothers should have formal visual assessment before school entry.
Summary
What was known before
-
Treatment of opioid use disorder in pregnancy with methadone maintenance treatment improves pregnancy outcomes.
-
Prenatal opioid exposure may lead to neonatal abstinence/opioid withdrawal syndrome (NAS/NOWS) and abnormal visual evoked potentials: longer-term sequelae are reported but research is confounded by polydrug misuse, tobacco smoking, alcohol and challenged home environment.
-
Impaired visual development including strabismus and nystagmus has been reported but no long-term cohort data are available.
What this study adds
-
Being born to a methadone-maintained opioid dependent mother almost doubled the risk of children failing visual assessment at 8–10 years.
-
Almost half of exposed children had strabismus and one in five had nystagmus. Visual problems were equally frequent in children who did and did not receive treatment for NAS/NOWS.
-
Prenatal methadone exposure should be considered in the differential diagnosis of nystagmus.
-
Findings support visual assessment prior to school entry for children with a history of prenatal exposure to opioids and/or other substances of misuse, and may infer a teratogenic effect of methadone.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
ASAM. The ASAM National practice guideline for the treatment of opioid use disorder: 2020 focused update. J Addict Med. 2020;14:1–91.
Mactier H, Hamilton R. Prenatal opioid exposure - increasing evidence of harm. Early Hum Dev 2020;150:105188.
Krans EE, Kim JY, Chen Q, Rothenberger SD, James AE III, Kelley D, et al. Outcomes associated with the use of medications for opioid use disorder during pregnancy. Addiction. 2021;116:3504–14.
Clinical Guidelines on Drug Misuse and Dependence Update 2017 Independent Expert Working Group. Drug misuse and dependence: UK guidelines on clinical management. 2017. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/673978/clinical_guidelines_2017.pdf.
Baldacchino A, Arbuckle K, Petrie DJ, McCowan C. Erratum: Neurobehavioral consequences of chronic intrauterine opioid exposure in infants and preschool children: a systematic review and meta-analysis. BMC Psychiatry. 2015;15:134.
Monnelly VJ, Hamilton R, Chappell FM, Mactier H, Boardman JP. Childhood neurodevelopment after prescription of maintenance methadone for opioid dependency in pregnancy: a systematic review and meta-analysis. Dev Med Child Neurol 2019;61:750–60.
Lee SJ, Bora S, Austin NC, Westerman A, Henderson JMT. Neurodevelopmental outcomes of children born to opioid-dependent mothers: a systematic review and meta-analysis. Acad Pediatr. 2020;20:308–18.
Ostrea EM, Knapp K, Tannenbaum L, Ostrea AR, Romero A, Salari V, et al. Estimates of illicit drug use during pregnancy by maternal interview, hair analysis, and meconium analysis. J Pediatr. 2001;138:344–8.
Lange S, Shield K, Koren G, Rehm J, Popova S. A comparison of the prevalence of prenatal alcohol exposure obtained via maternal self-reports versus meconium testing: a systematic literature review and meta-analysis. BMC Pregnancy Childbirth. 2014;14:127.
Perlstein MA. Congenital Morphinism: a rare cause of convulsions in the newborn. JAMA. 1947;135:633.
Chavez C, Ostrea E, Stryker J, Strauss M. Ocular abnormalities in infants as sequelae of prenatal drug-addiction. Pediatr Res. 1979;13:367–367.
Rosen TS, Johnson HL. Children of methadone-maintained mothers: follow-up to 18 months of age. J Pediatr. 1982;101:192–6.
Johnson HL, Diano A, Rosen TS. 24-month neurobehavioral follow-up of children of methadone-maintained mothers. Infant Behav Dev. 1984;7:115–23.
Nelson L, Ehrlich S, Calhoun J, Matteucci T, Finnegan L. Occurrence of Strabismus in infants born to drug-dependent women. Am J Dis Child. 1987;141:175–8.
Dominguez R, Vilacoro A, Slopis J, Bohan T. Brain and ocular abnormalities in infants with inutero exposure to cocaine and other street drugs. Am J Dis Child 1991;145:688–95.
Gaillard M-C, Borruat F-X. [New finding: transitory horizontal pendular nystagmus secondary to neonatal abstinence syndrome]. Klin Monbl Augenheilkd. 2002;219:317–9.
Gill A, Oei J, Lewis N, Younan N, Kennedy I, Lui K. Strabismus in infants of opiate-dependent mothers. Acta Paediatr. 2003;92:379–85.
Lloyd D, Myerscough E, Grampian Health Board. Substance misuse research: neonatal abstinence syndrome: a new intervention. A community based, structured health visitor assessment. Edinburgh: Scottish Executive; 2006. Available at: https://webarchive.nrscotland.gov.uk/3000/https://www.gov.scot/Publications/2006/09/04102243/0.
Mulvihill AO, Cackett PD, George ND, Fleck BW. Nystagmus secondary to drug exposure in utero. Br J Ophthalmol. 2007;91:613–5.
Hamilton R, McGlone L, MacKinnon JR, Russell HC, Bradnam MS, Mactier H. Ophthalmic, clinical and visual electrophysiological findings in children born to mothers prescribed substitute methadone in pregnancy. Br J Ophthalmol. 2010;94:696–700.
Gupta M, Mulvihill AO, Lascaratos G, Fleck BW, George ND. Nystagmus and reduced visual acuity secondary to drug exposure in utero: long-term follow-up. J Pediatr Ophthalmol Strabismus. 2012;49:58–63.
Tinelli F, Gamucci A, Battini R, Cioni G. Congenital nystagmus in two infants born from mothers exposed to methadone during pregnancy. Ital J Pediatr 2013;39:40.
Cornish KS, Hrabovsky M, Scott NW, Myerscough E, Reddy AR. The short- and long-term effects on the visual system of children following exposure to maternal substance misuse in pregnancy. Am J Ophthalmol. 2013;156:190–4.
Melinder A, Konijnenberg C, Sarfi M. Deviant smooth pursuit in preschool children exposed prenatally to methadone or buprenorphine and tobacco affects integrative visuomotor capabilities. Addiction. 2013;108:2175–82.
Traband A, Lambert J, Christiansen SP. Ocular comorbidities in children with neonatal abstinence syndrome. Investig Ophthalmol Vis Sci. 2014;55:4093.
Joy J, Allman A. G135(P) Chasing the welsh dragon: a review of the outcome of infants with neonatal abstinence syndrome over a 10 year period. Arch Dis Child. 2015;100:A59.2–A59.
Kivisto K, Tupola S, Kivitie-Kallio S. Prenatally buprenorphine-exposed children: health to 3 years of age. Eur J Pediatr. 2015;174:1525–33.
Konijnenberg C, Melinder A. Visual selective attention is impaired in children prenatally exposed to opioid agonist medication. Eur Addict Res. 2015;21:63–70.
Uebel H, Wright IM, Burns L, Hilder L, Bajuk B, Breen C, et al. Reasons for rehospitalization in children who had neonatal abstinence syndrome. Pediatrics. 2015;136:e811–e820.
Yoo SH, Jansson LM, Park H-J. Sensorimotor outcomes in children with prenatal exposure to methadone. J Aapos. 2017;21:316–21.
Merhar SL, McAllister JM, Wedig-Stevie KE, Klein AC, Meinzen-Derr J, Poindexter BB. Retrospective review of neurodevelopmental outcomes in infants treated for neonatal abstinence syndrome. J Perinatol. 2018;38:587–92.
Hall ES, McAllister JM, Wexelblatt SL. Developmental disorders and medical complications among infants with subclinical intrauterine opioid exposures. Popul Health Manag. 2019;22:19–24.
Self JE, Dunn MJ, Erichsen JT, Gottlob I, Griffiths HJ, Harris C, et al. Management of nystagmus in children: a review of the literature and current practice in UK specialist services. Eye. 2020;34:1515–34.
Auger N, Rheaume M-A, Low N, Lee GE, Ayoub A, Luu TM. Impact of prenatal exposure to opioids, cocaine, and cannabis on eye disorders in children. J Addict Med. 2020;14:459–66.
Rees P, Stilwell PA, Bolton C, Akillioglu M, Carter B, Gale C, et al. Childhood health and educational outcomes after neonatal abstinence syndrome: a systematic review and meta-analysis. J Pediatr. 2020;226:149–156.e16.
Hemmati Z, Conti AA, Baldacchino A. Ophthalmic outcomes in children exposed to opioid maintenance treatment in utero: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2022;136:104601.
Hughes PH, Barker NW, Crawford GA, Jaffe JH. The natural history of a heroin epidemic. Am J Public Health. 1972;62:995–1001.
Hughes PH, Rieche O. Heroin epidemics revisited. Epidemiol Rev. 1995;17:66–73.
Barrio G, Montanari L, Bravo MJ, Guarita B, Fuente L, de la, Pulido J, et al. Trends of heroin use and heroin injection epidemics in Europe: findings from the EMCDDA treatment demand indicator (TDI). J Subst Abus Treat. 2013;45:19–30.
McGlone L, Hamilton R, McCulloch DL, Boulton R, Bradnam MS, Weaver LT, et al. Neonatal visual evoked potentials in infants born to mothers prescribed methadone. Pediatrics. 2013;131:E857–E863.
McGlone L, Hamilton R, McCulloch DL, MacKinnon JR, Bradnam M, Mactier H. Visual outcome in infants born to drug-misusing mothers prescribed methadone in pregnancy. Br J Ophthalmol. 2014;98:238–45.
McGlone L, Mactier H. Infants of opioid-dependent mothers: Neurodevelopment at six months. Early Hum Dev 2015;91:19–21.
McLoone P. Carstairs scores for Scottish postcode sectors from the 2001 census.; 2004. Available at: https://www.gla.ac.uk/researchinstitutes/healthwellbeing/research/mrccsosocialandpublichealthsciencesunit/programmes/inequalities/healthinequalities/determinantsofhealthandhealthinequalitiesinscotland/carstairsscores/.
Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ. 2014;348:f7450.
Williams C, Northstone K, Howard M, Harvey I, Harrad RA, Sparrow JM. Prevalence and risk factors for common vision problems in children: data from the ALSPAC study. Br J Ophthalmol. 2008;92:959–64.
Pathai S, Cumberland PM, Rahi JS. Prevalence of and early-life influences on childhood strabismus: findings from the millennium cohort study. Arch Pediatr Adolesc Med 2010;164:250–7.
Sarvananthan N, Surendran M, Roberts EO, Jain S, Thomas S, Shah N, et al. The prevalence of nystagmus: the Leicestershire Nystagmus Survey. Investig Ophthalmol Vis Sci. 2009;50:5201–6.
Hertle RW, Dell’Osso LF. Fusion Maldevelopment Nystagmus syndrome. In: Nystagmus in infancy and childhood: current concepts in mechanisms, diagnoses, and management. New York: Oxford University Press; 2013. pp. 103–21.
Merhar SL, Jiang W, Parikh NA, Yin W, Zhou Z, Tkach JA, et al. Effects of prenatal opioid exposure on functional networks in infancy. Dev Cogn Neurosci. 2021;51:100996.
Monnelly VJ, Anblagan D, Quigley A, Cabez MB, Cooper ES, Mactier H, et al. Prenatal methadone exposure is associated with altered neonatal brain development. NeuroImage: Clin. 2018;18:9–14.
Tychsen L, Richards M, Wong A, Foeller P, Bradley D, Burkhalter A. The neural mechanism for latent (fusion maldevelopment) Nystagmus. J Neuro-Ophthalmol. 2010;30:276–83.
Jantzie LL, Maxwell JR, Newville JC, Yellowhair TR, Kitase Y, Madurai N, et al. Prenatal opioid exposure: the next neonatal neuroinflammatory disease. Brain Behav Immun. 2020;84:45–58.
Chin EM, Kitase Y, Madurai NK, Robinson S, Jantzie LL. In utero methadone exposure permanently alters anatomical and functional connectivity: a preclinical evaluation. Front Pediatr 2023;11:1139378.
McLean RJ, Windridge KC, Gottlob I. Living with nystagmus: a qualitative study. Br J Ophthalmol. 2012;96:981–6.
Yeoh SL, Eastwood J, Wright IM, Morton R, Melhuish E, Ward M, et al. Cognitive and motor outcomes of children with prenatal opioid exposure: a systematic review and meta-analysis. JAMA Netw Open. 2019;2:e197025.
Andersen JM, Høiseth G, Nygaard E. Prenatal exposure to methadone or buprenorphine and long-term outcomes: a meta-analysis. Early Hum Dev. 2020;143:104997.
Boardman JP, Mactier H, Devlin LA. Opioids and the developing brain: time to rethink perinatal care for infants of opioid-dependent mothers. Arch Dis Child—Fetal Neonatal Ed. 2022;107:98–104.
Bann CM, Newman JE, Poindexter B, Okoniewski K, DeMauro S, Lorch SA, et al. Outcomes of Babies with Opioid Exposure (OBOE): protocol of a prospective longitudinal cohort study. Pediatr Res. 2023;93:1772–9.
Acknowledgements
We thank the children and their families; the research nursing and administrative team, Helen Bannister, Emily Blyth, Karen Duffy, Annmarie Jordan, Barry Milligan, Leeanne Milne, Ashleigh Neil, Elizabeth Waxman; Professor Ben Thompson, Ravi Purohit, and Sam B. Hutton for advice and support with eye movement recordings and analysis; Medical Illustration Services NHS Greater Glasgow & Clyde, Dr Sarah Brown and Dr Raja Mukherjee for facial photography and advice and support with its analysis; Dr Kathleen Vancleef and Professor Jenny Read for advice and sharing Asteroid stereotest software; Richard Davison, optometrist; Dr Richard Boulton for database support; Professor Alex McConnachie for statistical advice; Dr Laura McGlone for invaluable VIDI study management.
Funding
This work was funded by a joint grant from Action Medical Research, Horsham, UK and the Chief Scientist Office, Edinburgh, UK (ref GN2493) and supported by The RS Macdonald Charitable Trust.
Author information
Authors and Affiliations
Contributions
RH conceived the study, substantially contributed to the overall design, analysis and interpretation of data and drafted the final manuscript. AM substantially contributed to the design of the study. LB substantially contributed to the design of the study and data acquisition. AC substantially contributed to data analysis. EI substantially contributed to data interpretation. DLM substantially contributed to study conception, study design, and data interpretation. AMcN and KM substantially contributed to data acquisition. KMS substantially contributed to study conception, study design, and data interpretation. JWW substantially contributed to the design of the study and data analysis and data acquisition. HM conceived the study and substantially contributed to study design and data interpretation. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamilton, R., Mulvihill, A., Butler, L. et al. Impaired vision in children prenatally exposed to methadone: an observational cohort study. Eye 38, 118–126 (2024). https://doi.org/10.1038/s41433-023-02644-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02644-3